Bohr s atomic model overview importance
Table of Contents
Table of Contents
Are you struggling to understand how to draw a Bohr model? Perhaps you’re a student learning about atoms for the first time or a science enthusiast who wants to enhance their understanding of atomic structures. Whatever your reason for wanting to learn, this blog post will guide you through the process of drawing a Bohr model.
The Pain Points of Drawing a Bohr Model
Many students find it difficult to understand how electrons move around the nucleus in an atom. Without a clear understanding of this concept, it’s challenging to draw a Bohr model accurately. Additionally, some learners may struggle to identify the number of electrons in each electron shell, known as energy levels. This identification is essential to creating a correct Bohr model.
How to Draw a Bohr Model
Drawing a Bohr model can be a simple process if you understand the basic concepts. The first step is to identify the number of protons and neutrons in the atom’s nucleus. The atomic number of the element represents the number of protons, and the element’s mass number represents the sum of protons and neutrons. Next, identify the number of electrons present in each shell or energy level. Keep in mind that the first energy level can hold a maximum of two electrons. The second energy level can hold up to eight electrons, and the third energy level can hold up to 18 electrons. Once you have the number of electrons identified, place them in the appropriate energy level, starting from the innermost first one.
Summary of How to Draw a Bohr Model
To draw a Bohr model, identify the element’s number of protons and neutrons in the nucleus. Then, determine the number of electrons present in each shell, keeping in mind that each shell has a maximum number of electrons it can hold. Finally, start placing the electrons in the correct shell, starting from the innermost one.
How to Draw a Bohr Model – A Personal Experience
My first experience with drawing a Bohr model was during my chemistry class in high school. I found it challenging to understand the concept of energy levels and the electron placement in each shell. However, my teacher used a great analogy to explain it. She related the electron’s energy levels to the floors in a building. Just as a building’s first floor has a maximum occupancy of two people, the first energy level of an atom can hold only two electrons. Similarly, just as the fifth floor may have more people than the second, the fifth energy level or shell can hold more electrons than the second.
With this analogy, I finally understood the concept of energy levels and how to identify the number of electrons that each shell could hold. The Bohr model then became relatively easy to draw. Like my teacher, I encourage you to find analogies or real-life examples to better understand the concept of drawing Bohr models.
Electron Shells in More Detail
Electron shells are the energy levels where electrons orbit or move around the atom. There are up to seven energy levels or shells around the nucleus, with the first level being the closest to the nucleus. Electrons fill the available energy levels in a specific order, which helps us determine their valence, or bonding, properties. Scientists use the order of filling energy levels to predict the characteristics of an element.
Valence Electrons and their Significance
Valence electrons are the electrons present in the outermost shell or energy level of an atom. These electrons are significant because they determine how an atom bonds with other atoms to form molecules. The number of valence electrons also determines the chemical and physical properties of an element. Elements in the same group, or column, of the periodic table have similar electron configurations, meaning they have the same valence electrons and similar bonding properties.
Common Mistakes to Avoid when Drawing a Bohr Model
While drawing a Bohr model, there are a few mistakes that you could make. One common mistake is placing too many electrons in a single energy level. This error is prevalent when students misinterpret the maximum number that each energy level can hold. Another mistake is forgetting to label the total number of protons and neutrons in the nucleus. This omission makes it challenging to determine the element that the atom represents.
Question and Answer
Q: How many electrons can fit in the third energy level?
A: The third energy level can hold up to 18 electrons.
Q: What is the correct order for filling the electrons in Bohr’s model?
A: Electrons follow the order 2,8,18,32 with regards to the number of electrons that energy levels hold.
Q: Why are Bohr’s models significant?
A: Bohr’s models are significant because they help us understand and predict the properties of atoms and molecules, including their bonding and reactivity.
Q: How many electrons can the first energy level hold?
A: The first energy level can hold a maximum of two electrons.
Conclusion of How to Draw a Bohr Model
Drawing a Bohr model might seem challenging at first, but with the right approach, it becomes a simple process that helps you better understand the fundamental concepts of atomic structures. Follow the steps we’ve provided here and avoid the common mistakes, and you’ll be on your way to drawing accurate Bohr models in no time!
Gallery
How To… Draw Bohr Models - YouTube

Photo Credit by: bing.com / bohr draw model diagram models sponsored links
Bohr’s Atomic Model — Overview & Importance - Expii
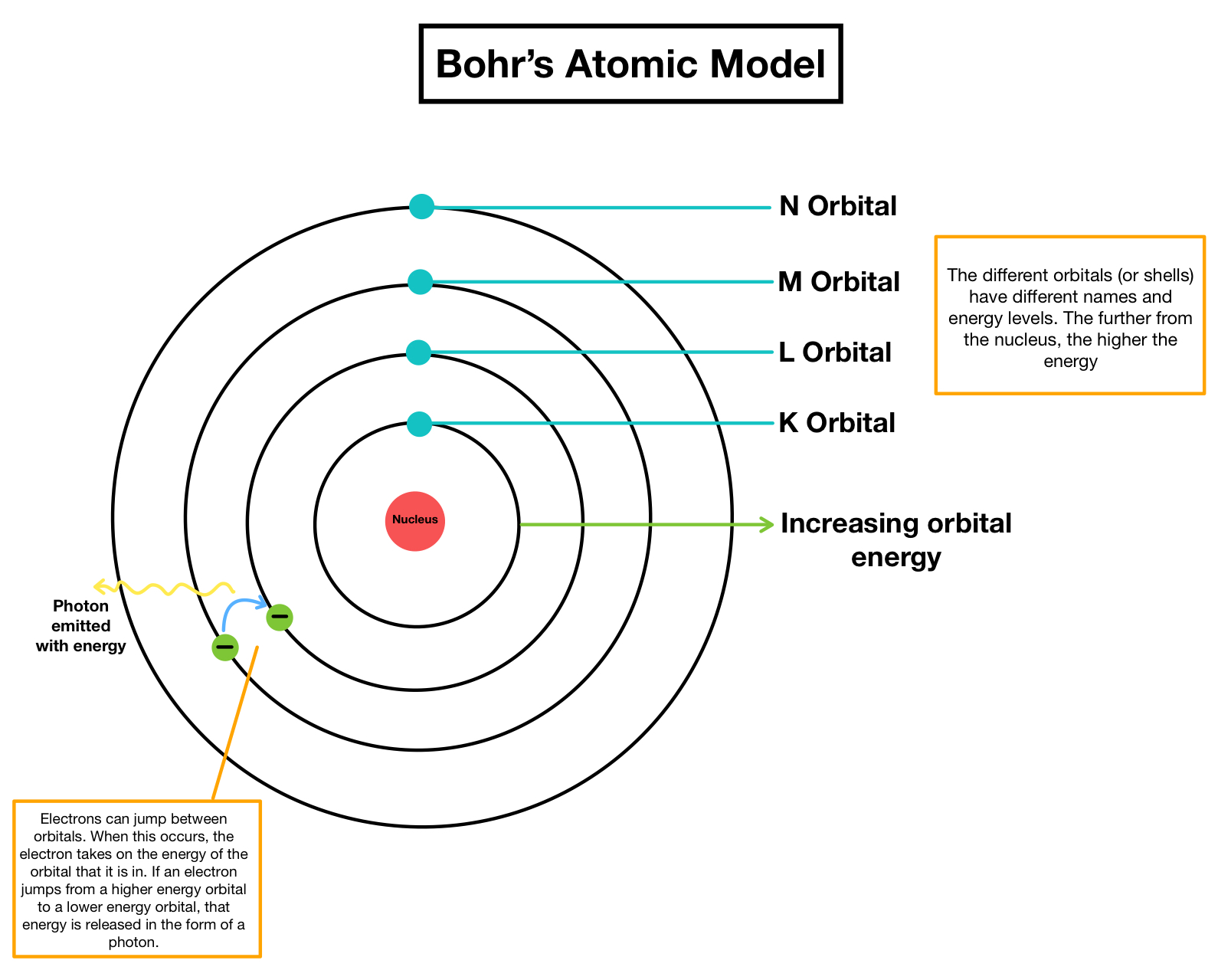
Photo Credit by: bing.com / bohr bohrs levels orbitals electron monahan
12 Best Images Of Bohr Model Worksheet - Bohr Model Worksheet Answers
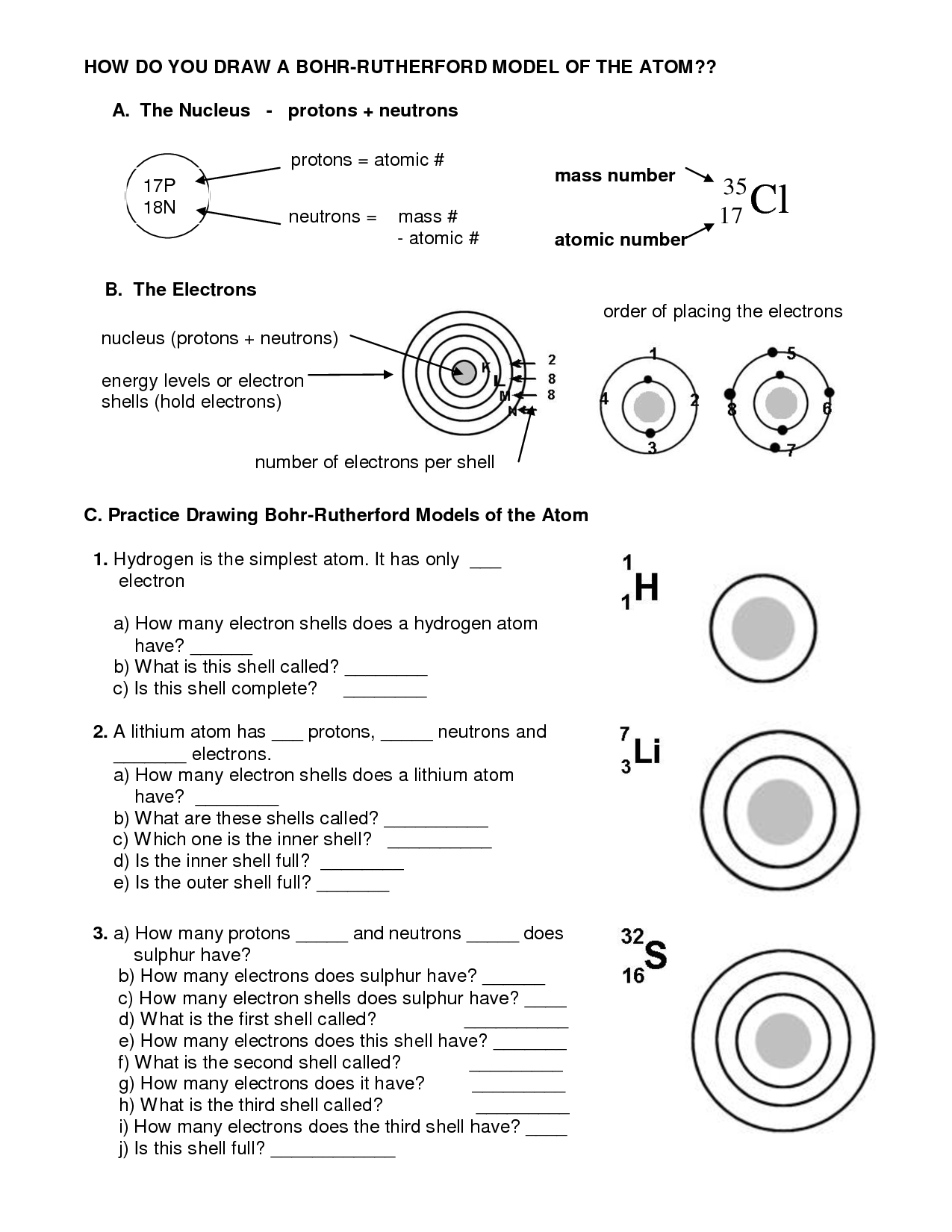
Photo Credit by: bing.com / bohr worksheet model chemistry draw rutherford science drawing diagrams electrons oxygen practice atoms atom answers key answer atomic diagram electron
The Lab Lads: Bohr Models!

Photo Credit by: bing.com / bohr argon diagram model rutherford diagrams models periodic table electrons structure valence lewis matter unit examples 2a dot october protons
Bohr Model Drawing Oxygen At GetDrawings | Free Download
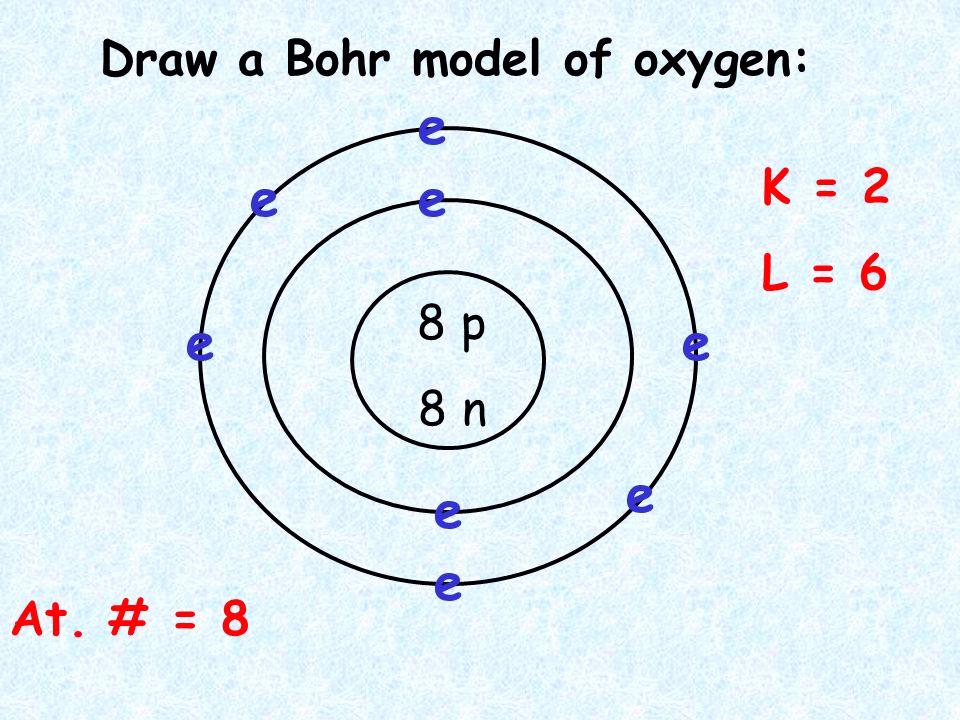
Photo Credit by: bing.com / bohr drawing oxygen model atomic diagram models getdrawings phosphorus different




